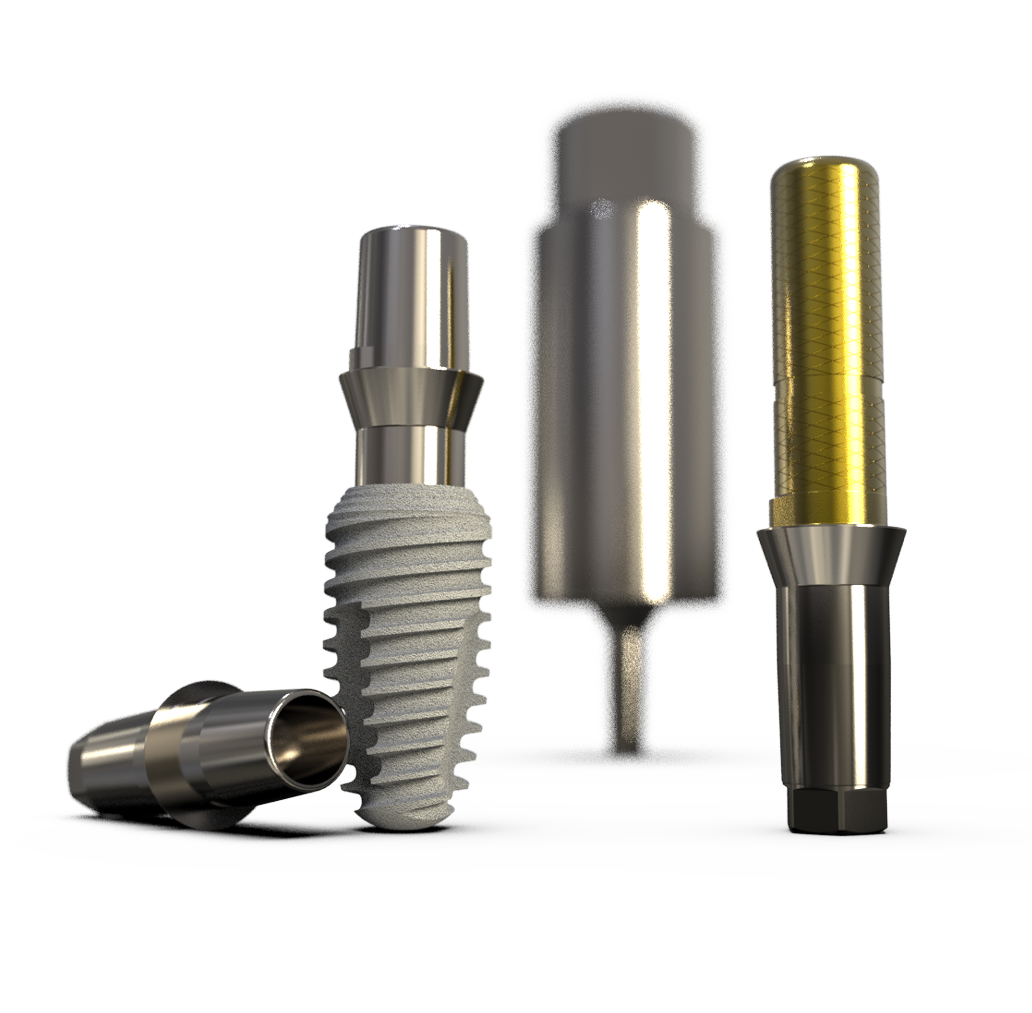
A Truly Sustainable Solution
The market average of common systems is characterized these days by epicrestal or crestal positioning. Despite platform switching, micro-movements of the abutment not only lead to screw loosening but also to bacterial colonization of the implant. These design-related movements initially lead to mucosal degradation, which brings aesthetic problems.
Subsequently, this leads to bone loss and the loss of primary stability. The implant may be lost in the medium term. In contrast, the proven philosophy of K3Pro® is subcrestal placement. Thanks to the absolutely bacteria-tight and micro-movement-free cone and the sloping shoulder, the implant heals deeply into the bone.
The gingiva adheres permanently. Through the form and force closure of the 1.5° cone, we achieve the best of both worlds: the surgical and prosthetic flexibility of the two-piece system while simultaneously distributing the load and stability as in one-piece implants. The distances to adjacent teeth or implants can be significantly reduced.

The Gold Standard in Augmentation Surgery
Allogeneic grafts are another success factor for the company. With Osteograft, Argon provides products that meet all the criteria of modern augmentative surgery for GTR and GBR applications. The high demands of the company's management
for maximum safety are met through collaboration with the German Institute for Cell and Tissue Replacement (DIZG) in Berlin. Osteograft grafts are produced by the DIZG from the German donor program and are approved as pharmaceuticals.
Extensive criteria for donor selection, a donor screening that exceeds EU directives, as well as a validated sterilization process for the removal or inactivation of viruses, bacteria, and fungi form the basis for the highest possible product confidence among practitioners and patients.


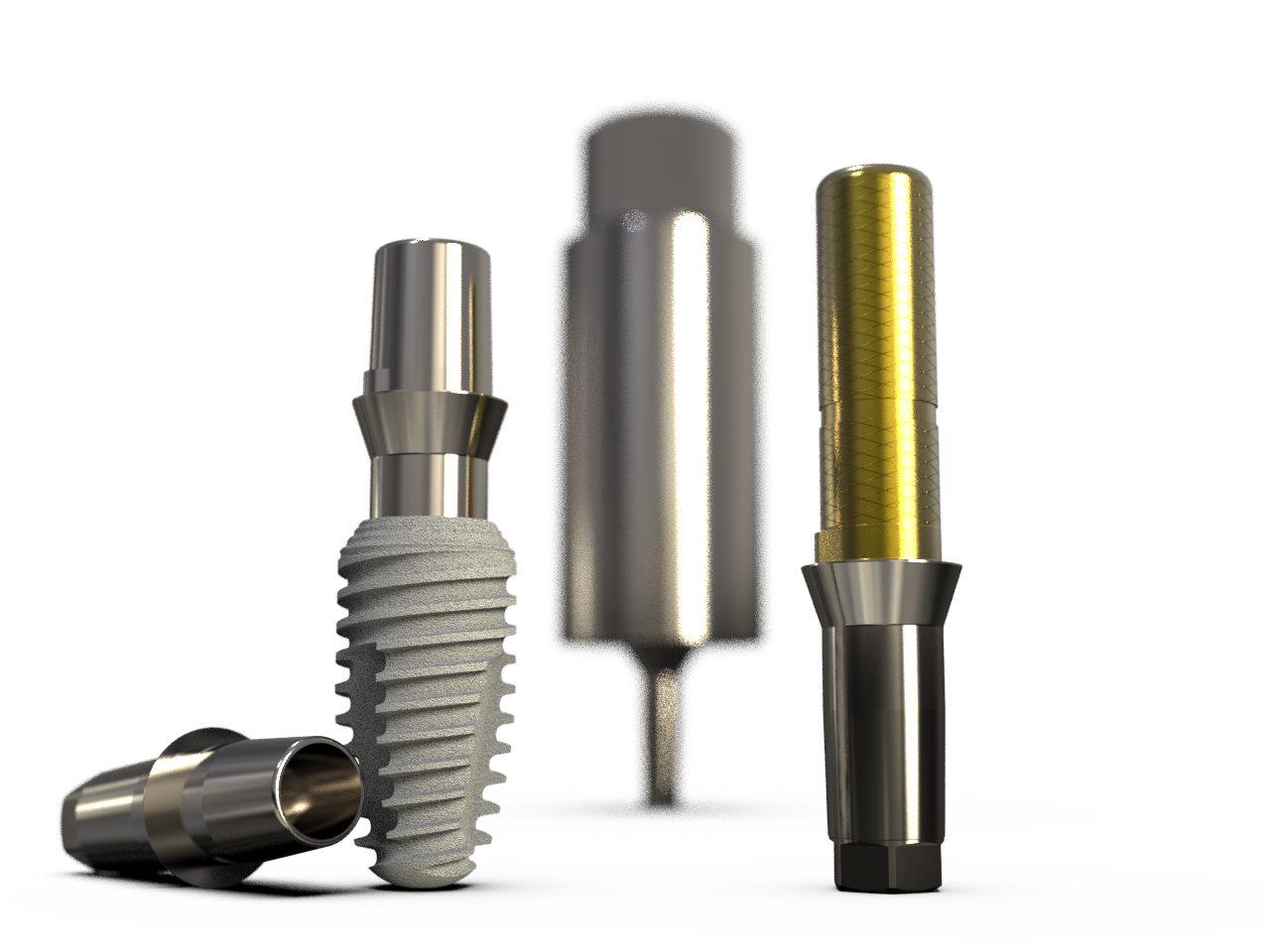
 K3Pro Implants
K3Pro Implants
 Implant Closure
Implant Closure
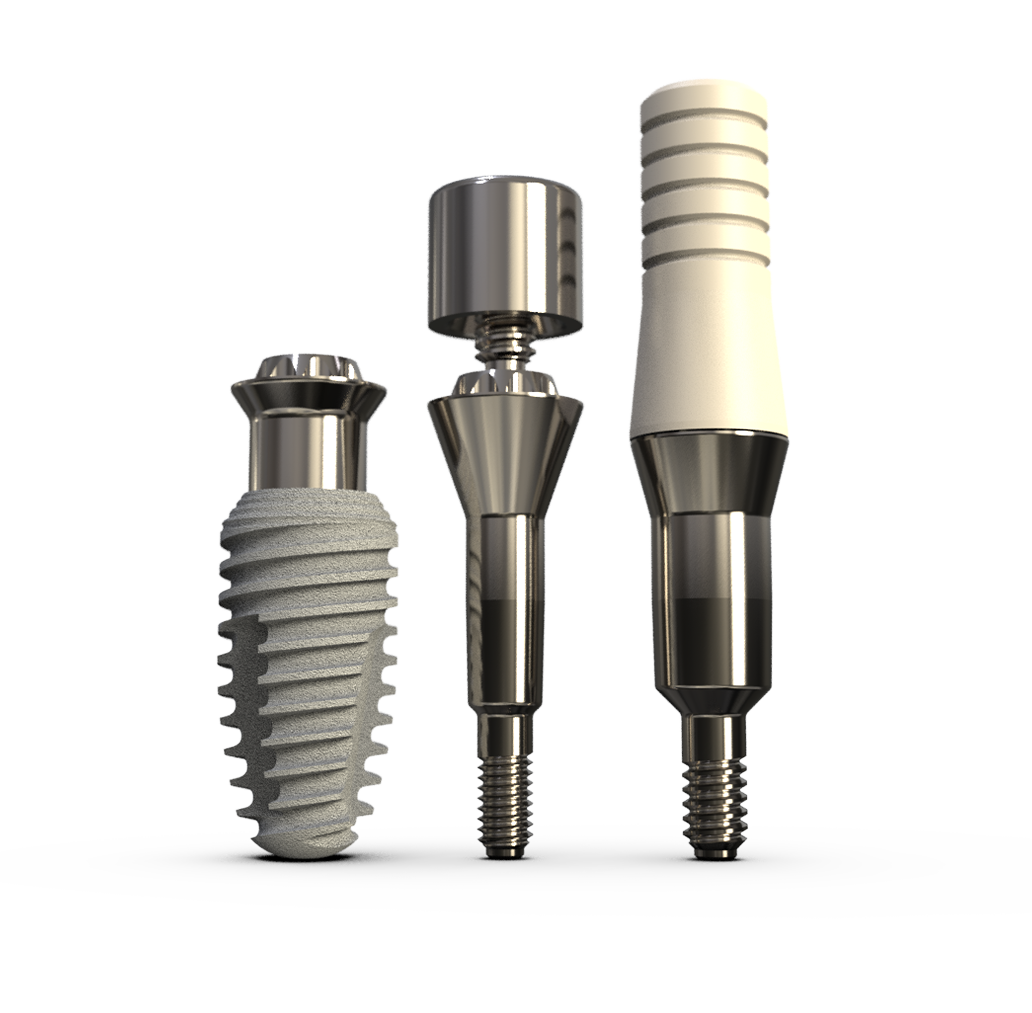 K3Pro Prosthetics
K3Pro Prosthetics
 K3Pro Lab Components
K3Pro Lab Components
 K3Pro Instruments & Accessories
K3Pro Instruments & Accessories
 Granules
Granules
 Blocks & Chips
Blocks & Chips
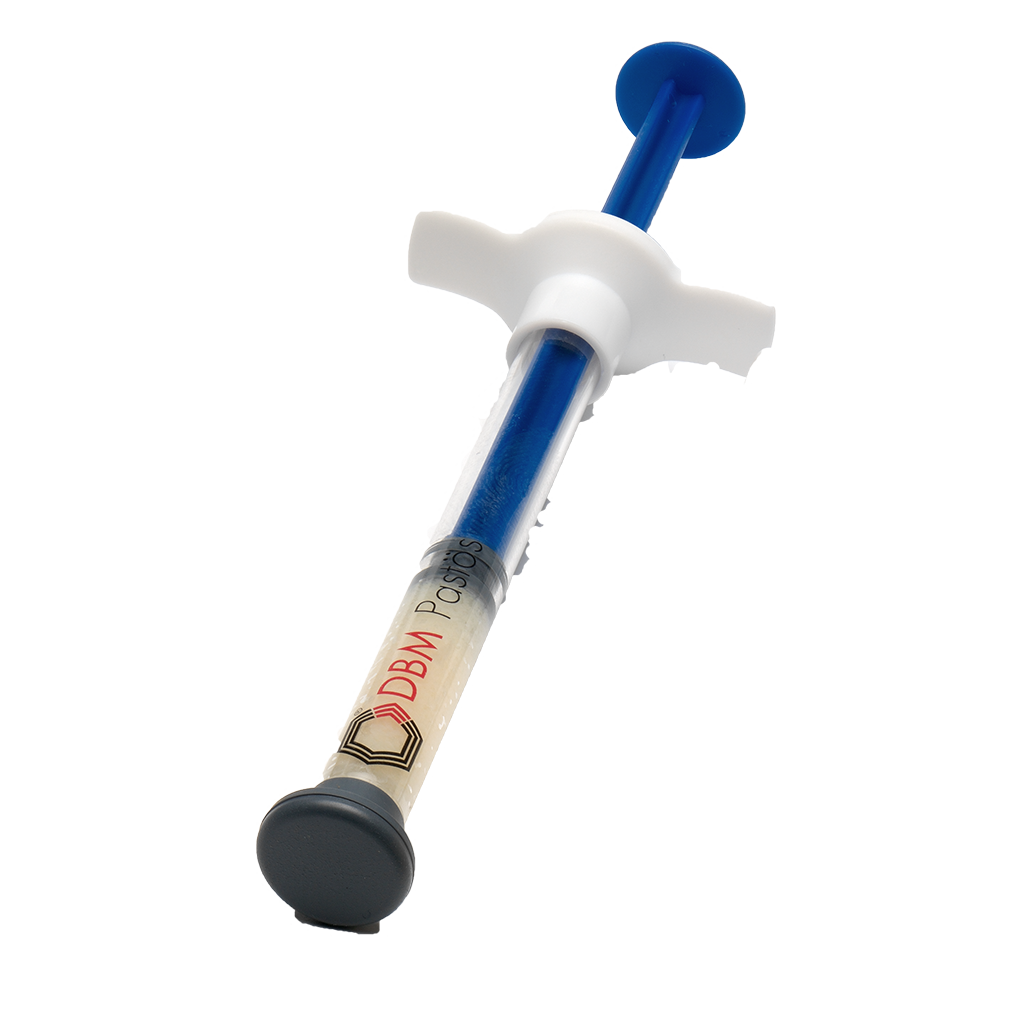 DBM Putty/ Pasty
DBM Putty/ Pasty
 Membranes
Membranes
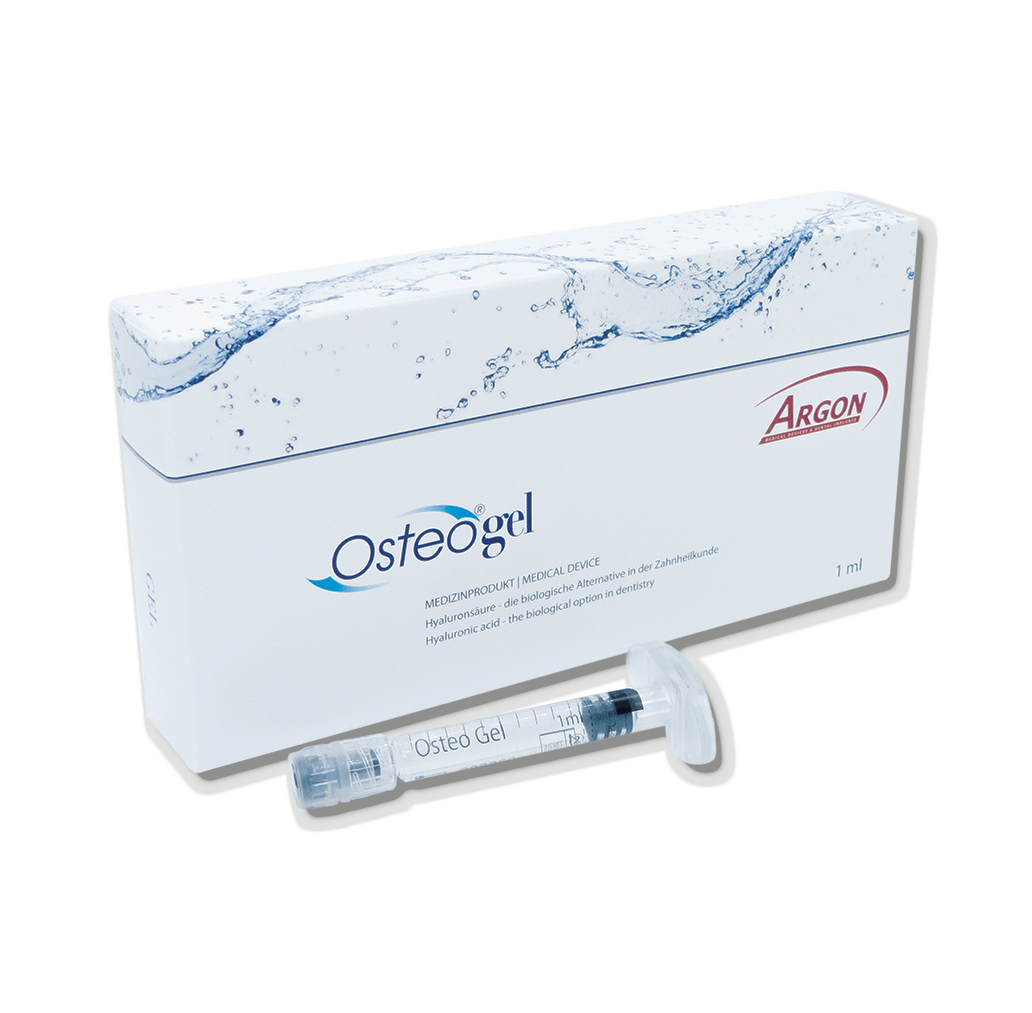 OsteoGel
OsteoGel